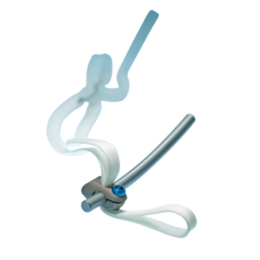
Ürün Açıklaması
Evrensel Kelepçe Spinal Fiksasyon Sistemi omurga anatomisi ve pedikül koruyucu bant geçiş tekniği ile çubuk arasındaki istikrarlı bir arayüz sağlar. Sonuç segmental stabilite sağlar ve sıkıştırma, oyalama, derotasyon ve çeviri pedikülleri ayırdığınız ve azaltırken implant / kemik temas stres sağlayan bir spinal implant sistemidir. Bu cerrahlar in situ çeviri, azaltma, oyalama ve sıkıştırma gerçekleştirmek için sağlayarak, kancalar, vidalar, teller birlikte çalışabilirsiniz. Ve bu Zimmer Omurga gelen beklediğiniz eğitim hizmeti ve uzmanlığı tarafından yedeklenir.
Özellikler
Evrensel Kelepçe spinal implant sistemi bir araya döşenmiş üç steril bölümden oluşmaktadır:
- Bir kelepçe
- Bir dokuma polyester bant
- Bir kilitleme vidası
Sistemin en büyük faydası basitliğidir. Bu omurganın ilerici posteromedial çeviri için basit bir süreç sağlar. Bu kullanıcının kancaların uyum ve sublaminar enstrümantasyon sadelik sunar. Zarif basit, Universal Clamp enstrümantasyon kaldıraç bu çok yönlü spinal implant dayanımlarına cerrahların spinal patolojiler çeşitli düzeltmek için izin verir. Tasarım yeniliği bir kanca ve bir tel ile daha 17 kat daha az olan 10 kat daha az temas stres sağlar.
ZS-SA0700-25_A
Description / Indications
- Spinal trauma surgery, used in sublaminar or facet wiring techniques.
- Spinal reconstructive surgery, incorporated into constructs for the purpose of correction of spinal deformities such as idiopathic and neuromuscular scoliosis in patients 8 years of age and older, adult scoliosis, kyphosis and spondylolisthesis.
- Spinal degenerative surgery, as an adjunct to spinal fusions.
Contraindications
- Disease conditions that have been shown to be safely and predictably managed without the use of fixation devices are relative contraindications to the use of these devices.
- Active systemic infection, or infection localized to the site of the proposed implantation, are contraindications to implantation.
- Severe osteoporosis is a contraindication because it may prevent adequate fixation.
- Suspected or documented metal allergy or intolerance.
- Inadequate tissue coverage over the operative site.
- In any situation where implant utilization would interfere with anatomical structures or expected physiological performance, such as impinging on vital structures.
- Severe fractures such that segments may not be maintained in satisfactory proximate reduction.
- Physical contact of the Universal Clamp System with dissimilar metals. The titanium clamp should only be used with titanium implants and the stainless steel clamp should only be used with stainless steel implants.
- Any entity or condition that compromises the possibility of fusion, i.e. such as cancer, kidney dialysis or osteopenia.
- Other relative contraindications include obesity, pregnancy, certain degenerative disease, and foreign body sensitivity. In addition, the patient’s occupation or activity level or mental capacity may be relative contraindications to this surgery. Specifically, some patients may, because of their occupation or lifestyle, or because of conditions such as mental illness, alcoholism or drug abuse, place undue stresses on the implant.
Warnings
- In the U.S.A., this product has labeling limitations.
- Potential risks identified with the use of this device system, which may require additional surgery, include: -Device component fracture. -Loss of fixation. -Non-union. -Fracture of the vertebra. -Neurological injury. -Vascular or visceral injury.
- Implants can break when subjected to the prolonged loading associated with delayed union or non-union. Internal fixation appliances are load sharing devices which are used to obtain an alignment until normal healing occurs. If healing is delayed or does not occur, the implant may eventually break due to fatigue. The degree or success of union, loads produced by weight bearing, and activity levels will, among other conditions, dictate the longevity of the implant. Patients should be fully informed of the risks of implant failure.
- Mixing metals can cause corrosion. There are many forms of corrosion damage and several of these occur on metals surgically implanted in humans. General or uniform corrosion is present on all implanted metals and alloys. The rate of corrosive attack on metal implant devices is usually very low due to the presence of passive surface films. Dissimilar metals in contact, such as titanium and stainless steel, accelerate the corrosion process of stainless steel and more rapid attack occurs. The presence of corrosion compounds released into the body system will also increase. Internal fixation devices, such as rods, hooks, wires, etc. which come into contact with the UNIVERSAL CLAMP System, must be made from like or compatible metals.
- In selecting patients for internal fixation, the following factors can be of extreme importance to the eventual success of the procedure:
Precautions
- SURGICAL IMPLANTS MUST NEVER BE REUSED. An explanted implant should never be re-implanted. Even though the device appears undamaged, it may have small defects and internal stress patterns, which may lead to early breakage.
- CORRECT HANDLING OF THE IMPLANT IS EXTREMELY IMPORTANT. Refer to the UNIVERSAL CLAMP System Surgical Technique for instructions for implantation.
- REMOVAL OF THE IMPLANT AFTER HEALING. Implants can loosen, fracture, corrode, migrate, possibly increase the risk of infection, cause pain, or stress shield bone even after healing, particularly in young, active patients. The surgeon should carefully weigh the risks versus benefits when deciding whether to remove the implant. Implant removal should be followed by adequate postoperative management to avoid refracture. If the patient is older and has a low activity level, the surgeon may choose not to remove the implant thus eliminating the risk involved with a second surgery.
- ADEQUATELY INSTRUCT THE PATIENT. Postoperative care and the patient’s ability and willingness to follow instructions are one of the most important aspects of successful bone healing. The patient must be made aware of the limitations of the implant and that physical activity and full weight bearing have been implicated in device failure. The patient should understand that a metallic or polyester implant is not as strong as normal, healthy bone and will fracture if excessive demands are placed on it in the absence of complete bone healing. An active, debilitated, or demented patient who cannot properly use weight-supporting devices may be particularly at risk during postoperative rehabilitation.
- Additional fixation is required at the cephalad and caudal ends of the construct in scoliosis surgery, especially in case of obesity, extreme kyphosis or muscular weakness, except where additional fixation would increase the risk to the patient.
Possible Adverse Effects
- Non-union, delayed union
- Disassembly, fraying, kinking, loosening, bending or breakage of any or all of the UNIVERSAL CLAMP System implant components
- Metal sensitivity, polyester sensitivity or allergic reaction to a foreign body
- Infection
- Foreign body reaction to the implants including possible tumor formation
- Pain, discomfort, or abnormal sensations due to the presence of the device
- Pressure on the skin from component parts, where there is inadequate tissue coverage over the implant causing skin irritation
- Loss of proper spinal curvature, correction height and/or reduction
- Implants cutting through soft osteoliorotic, osteolienic or cancellous bone
- Bone forming around the impant making removal difficult or impossible
- Cessation of growth in the operrated portion of bone
- Decrease in bone density due to stress shielding
- Vascular and/or nerve damage due to surgical trauma or presence of the device. Neurological difficulties including bowel and/or bladder dysfunction, impotence, retrograde ejaculation, and paraesthesia
- Bursitis
- Dural leak
- Paralysis
- Death
- Erosion of blood vessels due to the proximity of the device, leading to hemorrhage and/or death