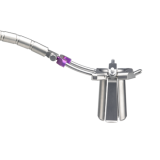
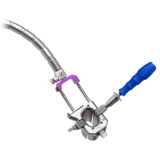
Product Description
The ARAS Retractor provides a streamlined access solution that maximizes direct visualization in MIS procedures.
Features
- ARAS provides direct visualization that adapts to address anatomical challenges and surgeon preferences through a less invasive, muscle-splitting approach
- The ARAS Retractor split-tube design expands via smooth, self-locking rails to achieve the desired exposure
- Curved rails on the ARAS retractor provide greater distal exposure with a smaller incision while matching the lordosis of the lumbar spine
ZS-SA0700-03_A
Indications
The ARAS Retractor Instrumentation is intended to provide surgical access to the thoracic and lumbar spine from a posterior and posterior lateral approach.
Contraindications
- Infection – overt or active
- Fever
- Local inflammation
Warnings
Misuse of the instrument may inhibit or prevent proper functioning and may cause injury to the patient or operator.
- Do not use the instrument for any purpose for which it is not intended.
- Ensure that the instrument is clean and sterile prior to use.
- If damaged do not reuse; replace.
- Instruments designated for single use only must not be reused and must be properly disposed of.
Precautions
It is strongly recommended that the patient be informed of the risks associated with surgical procedures and components.
- Carefully read all instructions and be familiar with the surgical technique prior to use.
- Inspect all instruments prior to each use for wear, corrosion, loosening, ageing or fracture. The spacer cutter blade should be periodically replaced to maintain optimal cutting performance.
- If instruments are subjected to excessive loading, are damaged or are handled improperly, they may fracture, corrode, oxidize and show excessive wear or their functionality may be impaired.
- Neutral pH enzymatic and cleaning agents are recommended. Cleaning or disinfecting agents containing aldehyde, mercury, active chlorine, chloride, bromine, bromide, iodine, iodide, and potassium or sodium hydroxide can accelerate the degradation of these instruments and should not be used.
- Never tighten Universal joints or Rail Clamps without Frame Components or Retractors in the holes. Joints may collapse and be rendered unusable.
- All implants and some instruments are intended for single use only; refer to the product label to determine if the instrument is intended for single use only. Single use devices should not be re-used. Possible risks associated with re-use of single use devices include:
- Mechanical malfunction
- Transmission of infectious agents
Adverse Effects
Possible adverse effects associated with the use of orthopedic instruments are similar to those associated with any spine surgery, including:
- Infection
- Nerve damage
- Bleeding
- Scarring
- Pain